Shibori Cyanotypes
traditional printmaking with a scientific twist
MIT Museum
Collaborators: Yael Saiger and Ola Jachtorowicz


Shibori Cyanotypes
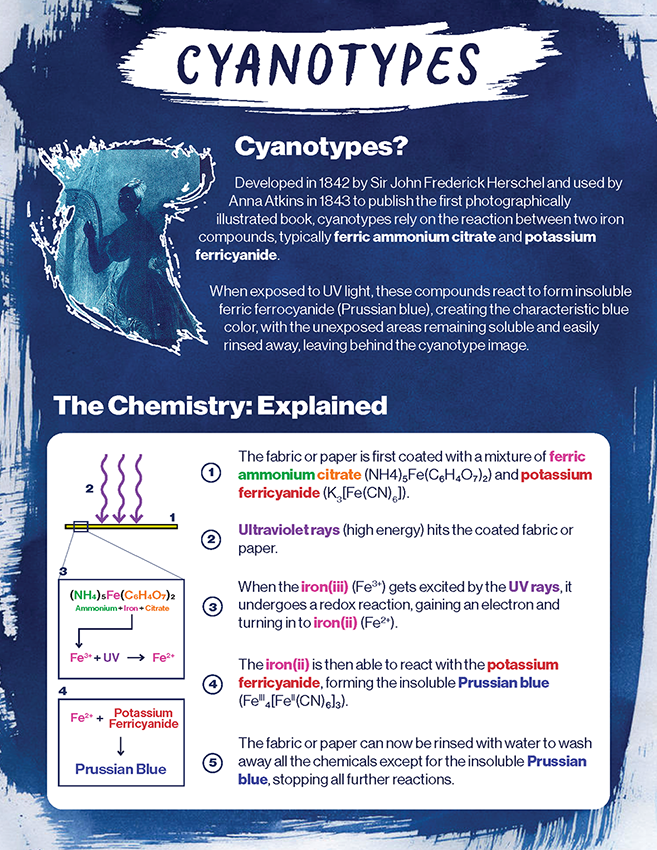
Cyanotypes were first discovered by Sir John Hershel in the 19th century using a mixture of ferric ammonium citrate and potassium ferricyanide. This discovery inspired early botanists such as Anna Atkins to use cyanotypes to capture intricate details of nature. Over time, the technique expanded beyond its scientific origins and became a means of artistic expression.
Shibori is a traditional Japanese resist-dyeing method that began in the 8th century as a way of enhancing the aesthetics of textiles. The name "shibori" originates from the Japanese word "shiboru," meaning to squeeze or wring. Through centuries, it diversified into various techniques, each producing unique patterns. Within the past two centuries, the method experienced a resurgence as artists and designers rediscovered its potential for creative exploration (e.g., Itchiku Kubota).
Shibori Cyanotypes combine these two techniques by merging the chemical processes of cyanotypes with the artistic methods of shibori to create patterned contemporary ink-prints.

Instructions
For these instructions, we used pretreated cyanotype fabric from Jacquard Products. Once the fabric is wrapped or pleated using the shibori techniques, place it under an ultraviolet (UV) lamp or under the sun and let it sit for around ten minutes. Optionally, flip the fabric around to expose all sides to UV rays. Once the fabric is exposed, thoroughly rinse the fabric under water and let the fabric dry. The color will start as a light yellow-green and as the dye oxidizes, a rich Prussian blue will develop.


The Chemistry
The fabric is first coated in a mixture of ferric ammonium citrate (in modern times, ferric ammonium oxalate is used) and potassium ferricyanide and left to dry. Once dry, the fabric can be exposed to ultraviolet rays, which have enough energy to initiate a photochemical reaction in the iron compounds (ferric). When the iron(III) gets excited by this energy, it undergoes a redox reaction, gaining an electron and turning into iron(II). The iron(II) is then able to react with the potassium ferricyanide, forming the insoluble ferric ferrocyanide (Prussian blue). The fabric is then washed to remove any water-soluble chemicals, stopping all further reactions.